Who we are
Blue Ridge Biomedical is a medical device company dedicated to improving outcomes in critical and postoperative care through clinically integrated diagnostics. We address gaps in current monitoring—where detection is delayed, intermittent, or burdensome—by designing technologies that provide earlier, actionable insights without disrupting clinical workflows. Founded by a multidisciplinary team of engineers and clinicians, we translate emerging biomarker research into practical point-of-care systems that support timely intervention, reduce complications, and improve patient care when it matters most.
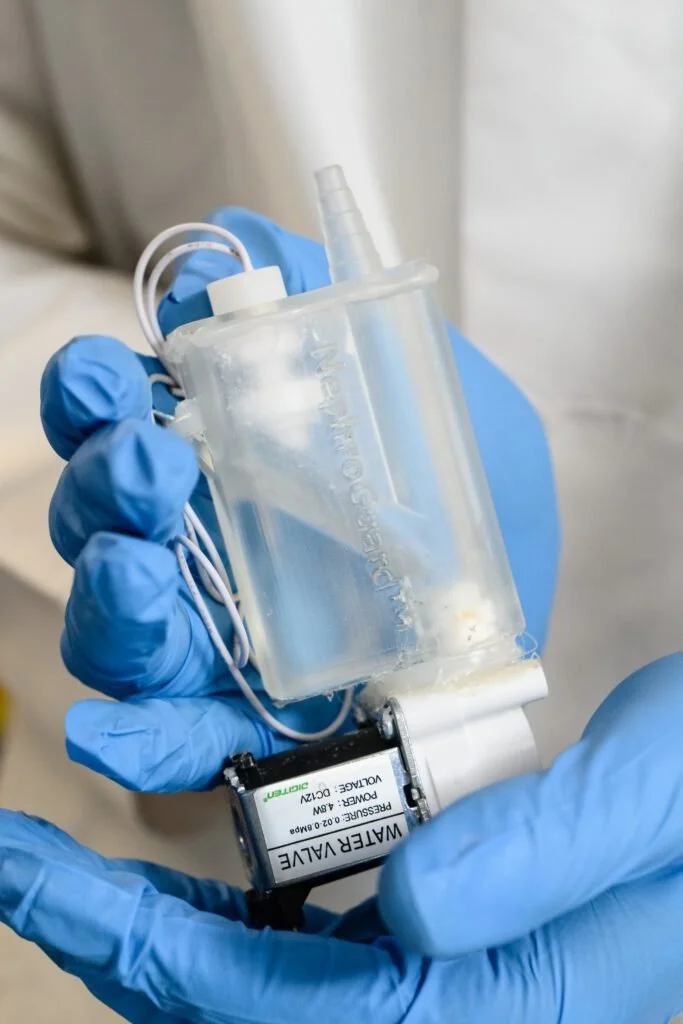
Our patent-pending product, NephroGuard, has received great recognition in several design and pitch competitions:
Atlantic Coast Conference InVenture 2025 — 2nd Place
NIH-DEBUT 2024 — NIDDK Kidney Technology Development Prize
Clemson SPARK Challenge 2024 — 1st Place
Clemson Launchpad Liftoff 2024 — 3rd Place
SC PDMA 2024 — 3rd Place

The problem
Current diagnostic methods are inaccurate and detect Acute Kidney Injury (AKI) 48-72 hours after onset, when irreversible kidney damage has already occurred
AKI occurs in up to 30% of cardiac surgery patients and can lead to increased ICU stays, chronic kidney disease, and dialysis [1]
The Solution
Continuous and automatic kidney damage biomarker monitoring to detect AKI within hours of onset
Compatible with all Foley catheters by connecting in-line
AI/ML‑enabled for accurate, automated diagnosis
[1] Vives, Marc, et al. "Cardiac surgery-associated acute kidney injury." Interactive cardiovascular and thoracic surgery 18.5 (2014): 637-645.